
ICP-OES (Element concentration in ppm)
S$28 per elementICP-MS(Element concentration in ppb)
S$38 per elementTesting Description
Inductively coupled plasma (ICP) is the main light source used for atomic emission spectroscopy and mass spectrometry, with ICP as the center, multiple detection units (each element with a detection unit) are installed around it, forming a multi-element analysis system. Using it as an excitation light source has the advantages of low detection limit, wide linear range, low ionization and chemical interference, and high accuracy and precision.
ICP-OES: Quantitative analysis of elements by accepting emission spectra of different wavelengths;
ICP-MS: The ions generated by the ion source are separated by the mass-to-charge ratio (m/z) through a four-stage rod, and the number of ions is calculated into the detector to detect the element concentration.
Sample requirements
1. For powder and bulk sample: Provide >100 mg. For liquid sample: Provide 5-10 mL, please contact us if it contains organic solvents.
2. Impurity elements such as Na, K, Ca, Mg, and Al are very common in the environment. If the content in the sample is low, it will lead to large errors. The influence of environmental interference can be reduced by increasing the sample amount. Please keep the shipping process clean and free from contamination and increase errors.
3 If the sample contains Li’ element and the content exceeds 2%, please note or contact the project manager to explain the situation. It needs to be buffered with water and then acid-dissolved, otherwise it may cause an explosion.
Examples
Calculation method:
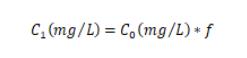
V₀: After the sample is digested, the volume at a constant volume, in milliliters (mL), 。corresponds to the data in (B) in the following table
f: Dilution factor, corresponding to the data in (E) in the following table
C₀: The concentration of the test solution element, in milligrams per liter (mg/L), the data is obtained by the instrument test, corresponding to the data in (D) of the following table
Note: The final result of the samples is calculated by formula above.
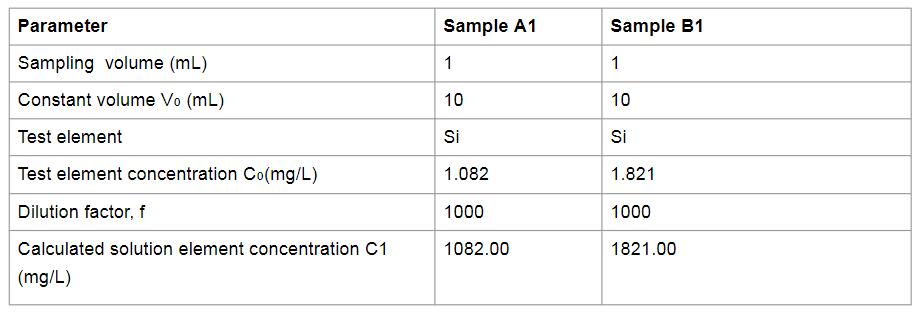
Test result:
FAQs



Resources