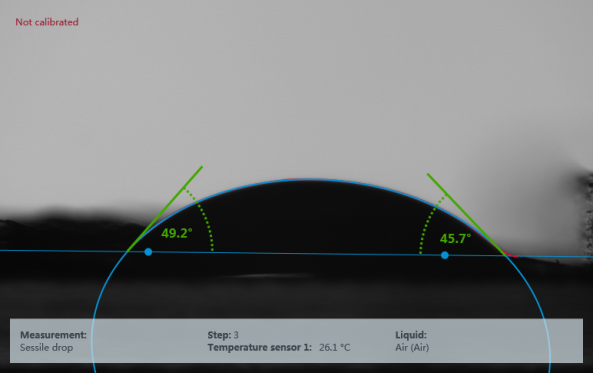
1. Definition of contact angle

Static contact angle: When the liquid reaches equilibrium on the solid surface, the angle between the boundary between the gas-liquid line and the boundary between the liquid and the solid is called the contact angle, which is the static contact angle. Dynamic contact angle: Generally for hydrophobic materials. If the three-phase interface front of the droplet is moving at the time of measurement, the contact angle value obtained by this measurement is called the dynamic contact angle. Forward/back angle and roll angle: There is a difference between the forward angle of the contact angle measured after the expansion of the solid-liquid interface and the backward angle of the measured value after the retraction, the forward angle is often greater than the reverse angle, the difference between the two is called the rolling angle, and the size of the rolling angle also represents the contact angle lag phenomenon of a solid surface. The observed static contact angle may be located anywhere within the range θA>θ >θR defined by the forward and backward shift contact angles. For a given three-phase system, the defined range of this static value is called contact angle hysteresis Δθ = θA-θR. Δθ is associated with the adhesion of the liquid on the solid, characterizing the degree of difficulty with which the droplet rolls on the surface of the solid. Surface tension: Due to the difference in density between the liquid phase and the gas phase, the molecules in the surface layer of the liquid are subject to a gravitational force pointing to the inside of the liquid phase and perpendicular to the interface, so that the liquid surface is like a tensioned elastic film, and there is a shrinkage tension on this film, so that the liquid surface has a tendency to shrink to the minimum. The shrinkage tension per unit length is called the surface tension. Surface free energy: Surface energy is also called surface free energy, liquid surface energy can be measured directly by instrumentation, and solid surface energy and solid-liquid interface energy can only be obtained indirectly by other methods.
2. How to test powder sample?

2. How to test powder sample? 2.1. Prepare a type of film sample by tablet pressing and test according to the film sample. The biggest factor affecting the test is whether the test surface is flat. 2.2. Direct testing (Washburn method) The gap between the solid powders is equivalent to a bundle of capillaries, and when the tube is inserted into the liquid to be measured, the liquid can spontaneously penetrate into the powder column due to capillary action. Capillary action depends on the surface tension of the liquid and the contact angle of the solid, so by determining the known surface tension liquid permeability in the powder column, the contact angle of the liquid to the powder can be obtained. There are also shortcomings in itself, that is, the equivalent capillary radius of the powder column is related to the particle size, shape and tightness of filling, and the linearity of the resulting curve is generally not ideal. In order to obtain relatively accurate results with this method, it is necessary to have the same degree of tightness of the powder sample and the column loading method and powder every time. On the other hand, when the gravity difference of the liquid relative to the Laplace pressure cannot be ignored, it will bring about a relatively large error.
3. What are the main test methods for surface tension?

3.1 Du Nouy Ring method: This is probably the most classic method of measuring surface/interfacial tension, and the surface/interfacial tension values of many liquids reported in the literature are measured using this method, and it can even be used in difficult cases where it is difficult to wet. A liquid film (similar to a soap bubble) is pulled out of the liquid with a ring initially immersed in the liquid and the force applied to raise the height of the ring is measured. 3.2 Wilhelmy Plate method: Also known as the hanging plate method, this is a very common measurement method, especially suitable for measuring surface tension for a long time, the measured amount is the force received by a plate perpendicular to the liquid level during the wetting process. With this method, only a sufficient volume of liquid needs to be supplied, and no other parameters are required. In fact, it is also possible to replace the plate with other geometric probes such as round rods, balls, etc., the measurement principle is the same, known as the improved William medley plate method. 3.3 Suspension drop method: is an interface shape analysis method, based on the analysis of the shape of an interface in a state of force equilibrium, is an optical analysis method suitable for the measurement of interfacial tension and surface tension, can also be measured at very high pressure and temperature, when measuring, it is necessary to know the two-phase substances and their density.